Twilight Fluorescence (TwiF) Microscopy
TwiF microscopy is a novel microscopy we have developed in 2017. It is capable of observing an object as thin as 1 nm, floating in solution. All you need to do is to mix your sample with a fluorescence dye and to place a drop of the mixture on a viewing glass plate. Then, focus the microscope as you would with an ordinary optical microscope. A world of nano-objects dancing in liquid is in front of you!
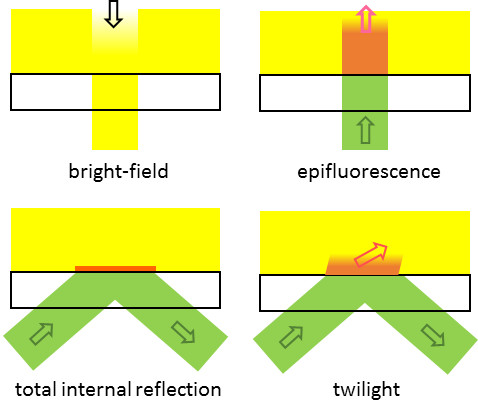
A unique feature of TwiF microscopy is a very weak illumination lighting only within a hundred micrometer of the glass surface. It is strongest at the glass surface, then diminishes as it propagates into the solution; resembling the twilight sky. With dim light, a very small change in contrast can be detected. You will see both a shadow of a bird hurrying to her home and a faintly blinking star at the same time in twilight. This image tickled my brain to develop TwiF microscopy.
As TwiF microscopy is just born, we do not know what kinds, how fine and what properties of a target we can observe. Let’s find out what kinds of a creature lives in the twilight zone.
Matsuno, Y.; Sato, Y.; Sato, H.; Sano, M. Direct Observations of Graphene Dispersed in Solution by Twilight Fluorescence Microscopy. J. Phys. Chem. Lett. 2017, 8, 2425-2431.
Physical Chemistry of Carbon Nanotube and Graphene

These nano-carbon materials possess excellent physical properties that can be applied in various fields of nanotechnology. We are interested in
・Dispersing nano-carbons in liquids and polymers
・Physical properties in solution
・Fabrication of thin films ・Thin film properties
・Thin film properties
・Interactions with biomolecules
・Development of new technologies integrated with polymer science
In particular, we aim for fundermental understanding of underlying principles that allow us to disperse nano-carbons in solution and to form thin films.
Gel Surfaces and Interfaces
Gel, like jelly and grease, is a state of matter having the properties of both a viscous liquid and an elastic solid. The present understanding of gel is based on the studies of a bulk region of gel where it is surrounded by the same gel continuum. In contrast, we hardly know anything about gel surfaces or interfaces with other medium.
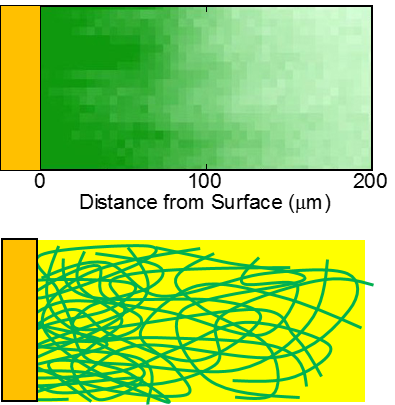
For example, a delicious fruit jelly in front of you is filled with sweet water inside. The water in gel is in the liquid state and can flow freely. If you cut the fruit jelly into many small pieces, each piece immediately becomes gel itself and no water ooze out. Why? If you answer "It's surface tension holding water inside the gel.", you have studied very well. But then, is it the only force that is holding water?
We have employed confocal Raman microscopy to answer these questions. The microscopy can map concertation profiles of each chemical species within the gel, including solvents. It is exciting to see new findings every time we take a look at the gel surfaces.